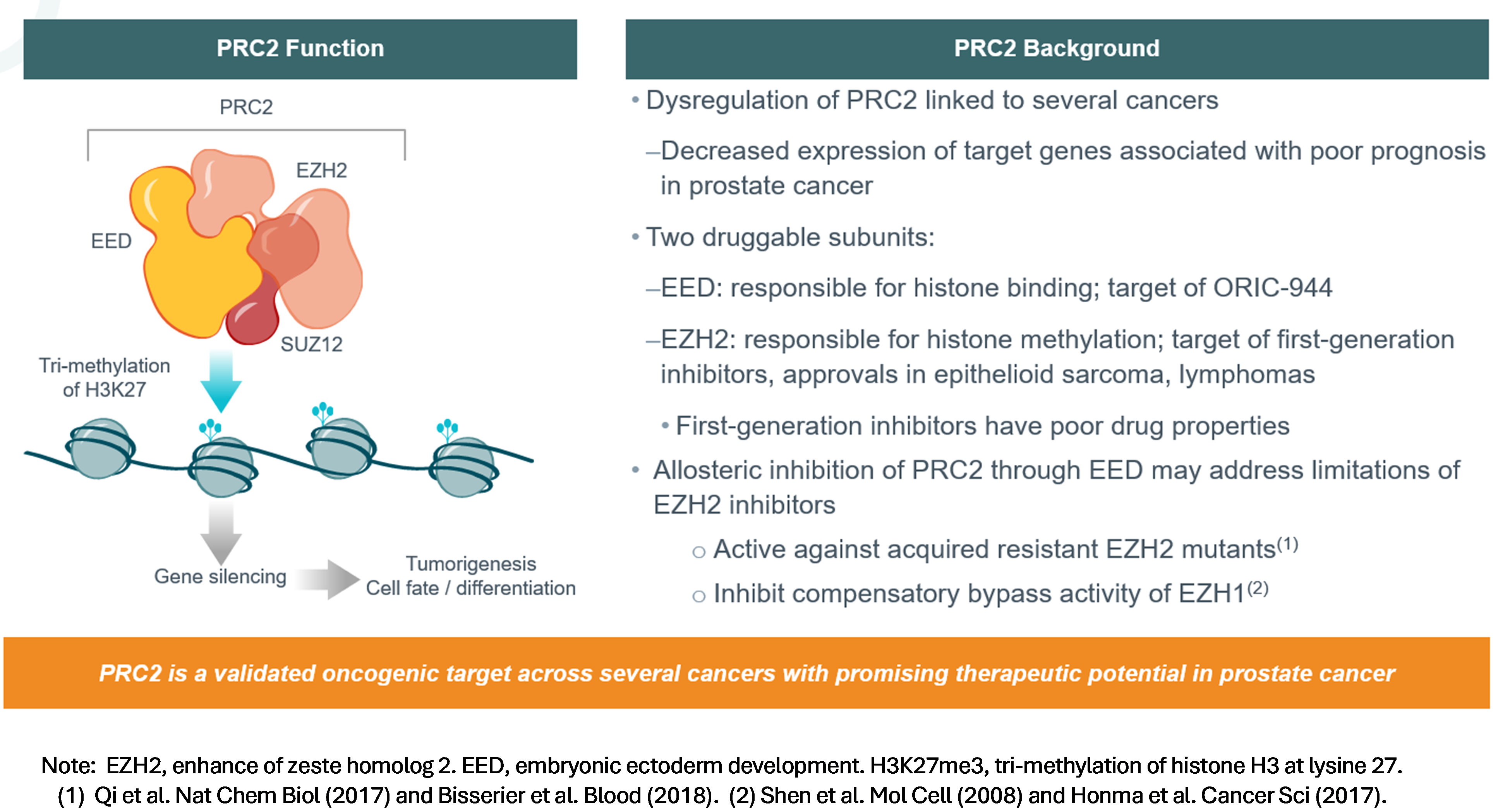
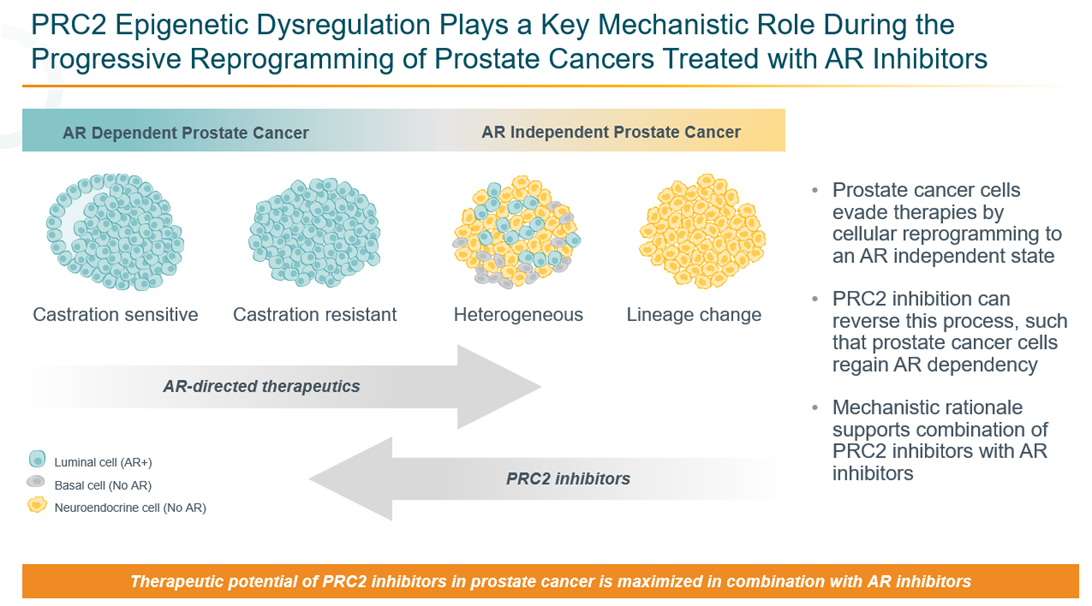
Now Enrolling: ORIC-944-01: An Open-Label, Phase 1/1b Study of ORIC-944 as a Single Agent or in Combination with an Androgen Receptor Pathway Inhibitor in Patients with Metastatic Prostate CancerORIC-944 is an investigational agent and not approved by the US Food and Drug Administration (FDA) or any other regulatory agency in any country. Mechanism of Action: ORIC-944 is a potent, highly selective, allosteric, orally bioavailable, small molecule inhibitor of PRC2 via binding the EED subunit.
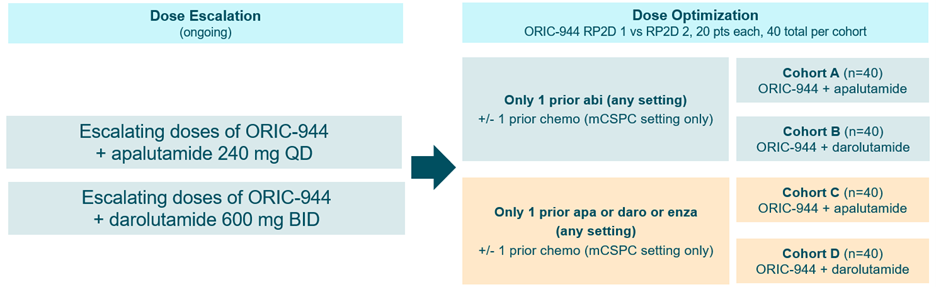
About the Study: This is a first-in-human, open-label, multicenter, dose escalation study of ORIC-944 as a single agent (Part I - COMPLETED) or in combination with an Androgen Receptor Pathway Inhibitor (ARPI) (apalutamide or darolutamide) (Part II) to establish the safety and preliminary antitumor activity of ORIC-944 as a single agent and in combination with the ARPIs in patients with metastatic prostate cancer. Part III (dose optimization) of the protocol will explore 2 potential dose levels of ORIC-944 selected from Part II in combinations with ARPIs to select the final RP2D for each combination across two separate patient populations. In Part II, escalating dose levels of ORIC-944 will be administered orally, QD in 28-day cycles in combinations with apalutamide 240 mg QD or darolutamide 600 mg BID. Combinations with abiraterone and enzalutamide may be initiated in the future. In Part III dose optimization, patients will be randomized 1:1 across two doses of ORIC-944 selected from Part II in combination initially with apalutamide or darolutamide, in each of two separate patient populations as shown in the study schema below.
Who may qualify for the study:
*This is not a complete list of criteria for participation and other study requirements may apply If you are a physician or health care provider interested in prostate cancer treatment, you can find more information about the trial at https://clinicaltrials.gov/study/NCT05315700?term=ORIC-114&rank=2. Additional Information:
Study Contact Information: Central Contact Person: ORIC Clinical Telephone: 650-388-5600 Email: [email protected]
|