|
Choose to be legendary.
Now Enrolling: LEGEND Phase 2 Sites & Patients
| ___ |
 |
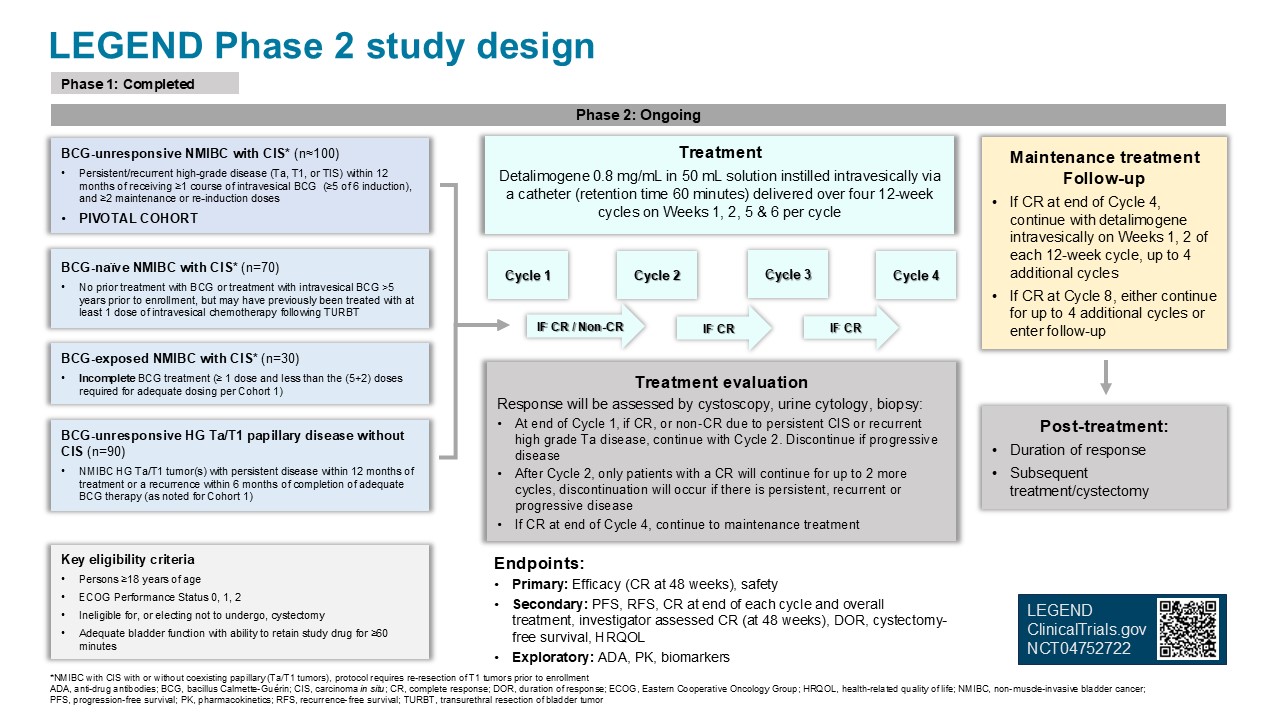
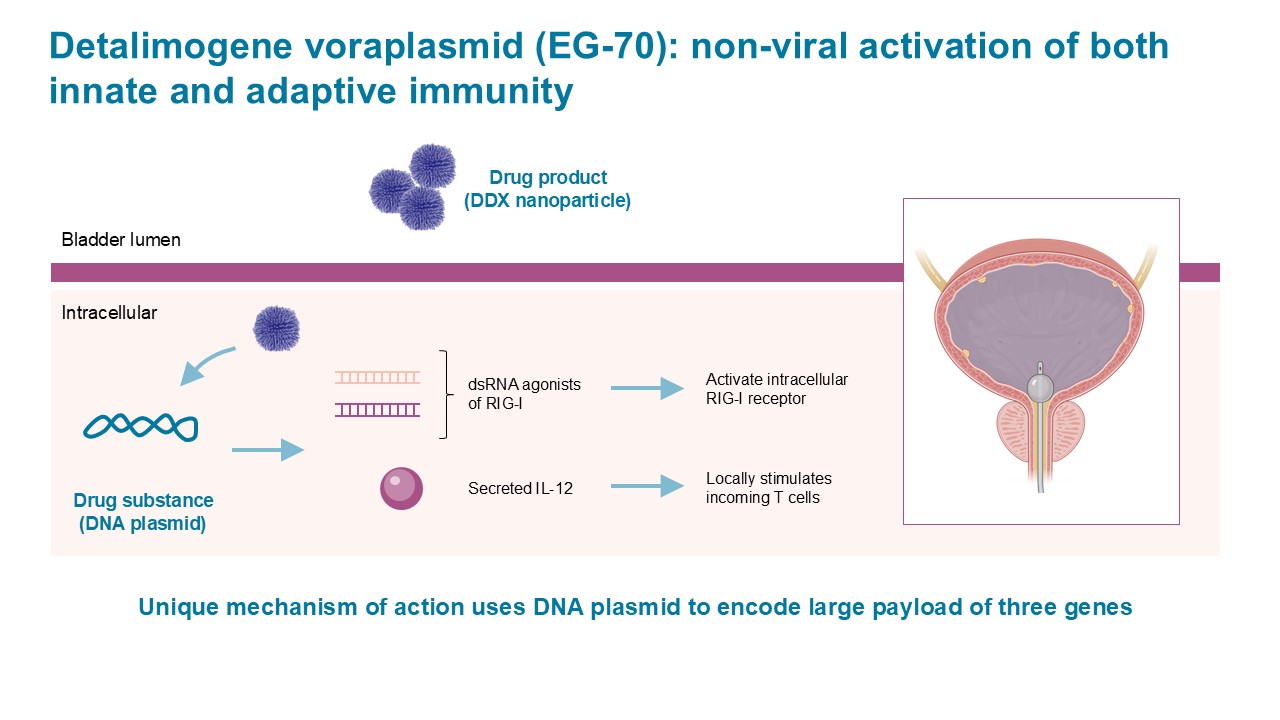
LEGEND: A Phase 1/2 study of EG-70 (detalimogene voraplasmid), a novel, investigational, non-viral intravesical gene therapy for patients with BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ (CIS)
About the LEGEND study
This study consists of two phases: a Phase 1 dose-escalation portion to establish safety and the recommended Phase 2 dose (completed), followed by a Phase 2 portion to establish the effectiveness of EG-70 (currently enrolling).
- EG-70, is an investigational non-viral gene therapy designed for intravesical administration
- All participants will receive EG-70 and will receive access to the study drug for up to 48 weeks (1 year), depending on their response to therapy.
| ___ |
 |
| |
Click the image above to enlarge. |
| ___ |
 |
| |
Click the images above to enlarge. |
What is the purpose of the Study?
The purpose of the LEGEND study is to evaluate if EG-70 can safely help the body destroy cancer cells in the bladder to delay or prevent surgical removal of the bladder for people with non-muscle invasive bladder cancer (NMIBC) who have not responded to Bacillus Calmette-Guérin (BCG) therapy.
*This is not a complete list of outcomes under evaluation in this clinical study.
Who may qualify for the LEGEND study?
- Diagnosed NMIBC with carcinoma in situ (CIS) that is BCG-unresponsive
- People who are 18 years of age or older
- People who are ineligible for or electing not to undergo, cystectomy
- ECOG performance status 0, 1, and 2
- Adequate renal (creatine clearance>30ml/min) and bladder function (ability to retain study drug for ≥ 60 minutes)
Key Exclusion Criteria
- History of partial cystectomy
- Any malignancy other than NMIBC diagnosed within 1 year of study entry (except basal or squamous cell skin cancers or noninvasive cancer of the cervix), or any malignancy that has required therapy for active disease within the last 12 months.
- Unresolved hydronephrosis due to ureteral obstruction,
- Unresolved vesicoureteral reflux or an indwelling urinary stent
- History of difficult catheterization that in the opinion of the Investigator will prevent study drug administration
- Active interstitial cystitis on cystoscopy
*This is not a complete list of criteria for participation and other study requirements may apply

LEARN MORE HERE
Contact enGene clinical trials
Phone Number: +18572991097
Email: [email protected]
|








